4.1 Bioreactor as a multivariable system
with nonlinear dynamics
Prior to discussing specific control applications, some general
features of bioreactors relevant to control applications should be mentioned.
The two main characteristics that are important to know before designing
a control system for a bioreactor are:
Multivariable system: As one would anticipate, control of a bioreactor will involve a system involving many variables.
Nonlinear dynamics: The control of a bioreactor is complicated by the fact that nonsteady state behavior is nonlinear. This has several consequences. Hysteresis is often observed. For example, a step increase in reactor feed rate in case of CSTBR (continuously stirred tank bioreactor) will result in a transient that will be different than when the corresponding equivalent step decrease in feed rate back to initial conditions is made. Moreover, multiple steady states are often observed for identical feed conditions, and in certain cases, exotic dynamics like limit cycles, oscillatory transients, long time lags may be exhibited.1
The reasons for the above mentioned behaviors are ultimately related to the complexities of living cells. Finally, many of the important variables which are desirable for monitoring and control are only measurable with large time lags or not measurable at all. This gives scope for accurate mathematical models and/or state estimation techniques. Fortunately, simple models and single input - single output feedback loops are available and work well in many cases.
4.2 Cell growth modeling in a batch reactor
The simplest way to model cell growth will be to consider
an unstructured, unsegregated model for cell growth. For this kind of model,
rx = dX/dt = mX (1)
where, rx = rate of cell generation (g/l-hr)
X = cell concentration (g/l)
m = specific growth rate (hr-1)
The most commonly used expression that relates the specific
growth rate of the cell to the substrate concentration is Monod's equation,
which is given as
m = mmaxS/(Ks + S)
(2)
where, m = specific growth rate (hr-1)
mmax = maximum specific growth (hr-1)
S = substrate concentration (g/l)
KS = saturation constant for substrate (g/l)
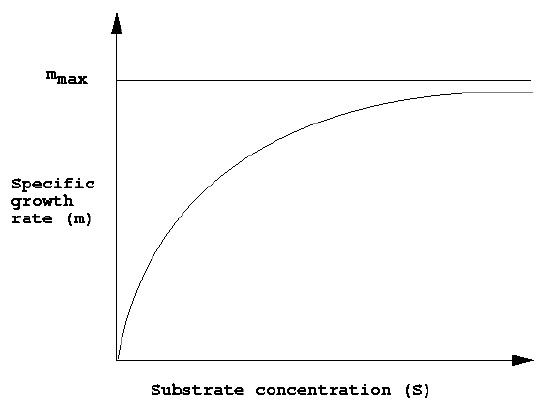
Figure 1 depicts the dependence of ? on S according to Monodís
equation. One should note that Monod's equation is empirical and does not
have any mechanistic basis.5
The equation is only valid for an exponentially growing culture under condition
of balanced growth. The equation does not
fair well in transient
conditions. Despite its simplicity
and no fundamental basis, it works

Figure 1. Monodís growth curve
surprisingly well in a large number of steady state and dynamic
situations. This characteristic has important implications in control of
bioreactors.
4.3 Continuous bioreactor dynamics
For a continuously fed bioreactor, the cells are continuously supplied substrate at growth limiting level, and hence they remain in the exponential phase. Since the cells remain in the exponential phase, Monod's equation can be applied. A cell balance on the reactor can be written as
FX - FXf + V(dX/dt) = rx
(3)
where, F = volumetric flow rate (l/hr)
X = cell concentration inside the reactor and in the outlet
stream (g/l)
Xf = cell concentration in the feed (g/l)
V = reactor volume (l)
rx = rate of cell generation (g/l-hr)
For a sterile feed (Xf = 0), and noting that the reaction rate can be written in terms of the specific growth rate (rx = mX), equation (3) can be reduced to
dX/dt = (m - D)X
(4)
where D = dilution rate = F/V (hr-1)
A balance on the substrate yields the following equation
FS - FSf + V(dS/dt) = rsV
(5)
where, F = volumetric flow rate (l/hr)
S = cell concentration inside the bioreactor and in the outlet
stream (g/l)
Sf = substrate concentration in the feed (g/l)
V = reactor volume (l)
rs = rate of substrate consumption (g/l-hr)
A yield parameter (Yx/s) is defined that relates the amount of cell mass produced per amount of substrate consumed, and is mathematically represented as
Yx/s = mass of cells produced/mass of substrate consumed = rx/-rs (6)
Combining equations (1), (5), and (6) yields
dS/dt = D(Sf - S) - mX/Yx/s (7)
The CSTBR (continuous stirred tank bioreactor) is now completely described by equations (4) and (7) with m given by equation (2). At steady state (with fixed Sf and D), the following are the values for m (specific growth rate), S (substrate concentration) and cell concentration (X)
m = D (8)
S = DKS/(mmax - D) (9)
X = Yx/s (Sf - S) (10)
There are a few characteristics of an open-loop CSTBR that are conceptually different from that of a chemical reactor which are important to know before any control system for a bioreactor can be designed. Figure 2 shows that D must be less than mmax for a realistic value of S to be achieved. The same conclusion can
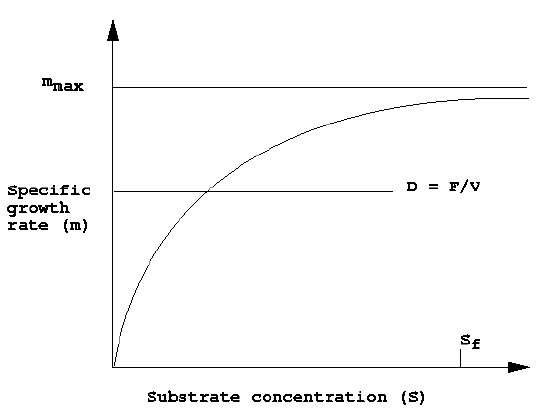
Figure 2. Relationship between dilution rate and specific growth rate for a steady state CSTBR
be derived by looking at the steady state solution of equation (4). The two solutions are equation (8) and
X = 0 (11)
The corresponding substrate concentration is
S = Sf (12)
Equation (11) and (12) define a situation called washout. This situation is encountered whenever the value of dilution rate equals or exceeds mmax. A rigorous discussion of washout would point to the fact that whenever m (Sf), i.e., m evaluated at Sf , is less than ?max, then the critical dilution rate for washout will occur at D = m (Sf), and not at D = mmax.1 The control algorithm should be completely aware of this unproductive state.
For the given set of equations, numerical solution is required since the system is described by two coupled nonlinear differential equations, i.e., equations (4) and (7). Linear control theory can be applied in only a limited sense, i.e., only near the steady state when the system model is linearized.1
Start up is an important consideration as well. The general procedure in the start up avoiding washout would be to initiate cell growth in a batch mode until the exponential phase is reached. At this point, the sterile feed would be started with a dilution rate such that D < m (Sf). A non washout steady state would be reached after a transient phase.1
4.4 Multiplicity and stability of steady states in a continuous bioreactor
Though the control loop of a CSTBR is simple, the system is complicated by the presence of multiple steady states and the stability considerations of these steady states. The following discussion will highlight these problems.
As already implied, the control design of a biological
reactor described by equation (4) and (7) should take into account the
nonlinear nature of these differential equations. Multiple critical points
are common with nonlinear systems. This has been shown earlier in the discussion
of washout. A systematic approach to an efficient control design will involve
1. calculation of the number of steady states
2. characterization of the nature of the steady states with
respect to their stability
3. design of appropriate control loops based on the results
from step 1 and step 2
4.4.1 Calculation of multiple steady states
Once the governing equations describing the system are in place, the steady states are found by replacing all time derivatives by zero. This can be done by inspection and algebric solution. For high order or complex models, a nonlinear root finding technique should be employed.1
4.4.2 Stability of a steady state
A steady state is stable if, for initial conditions near the steady state, all transients converge to it. If the transients diverge, steady state is called unstable. The diverging transients always end at some other stable state. Stability analysis of a steady state would involve whether the steady state under consideration is stable or not and the information about state - to - state transitions in case of unstable steady states.1
The information about the stability and local dynamics
of the steady states is accomplished through linear stability analysis.
It should be borne in mind that the results of the linear stability analysis
are good only near the steady state. For general (nonlocal) behavior and
information about state - to - state transitions, generation
of the phase plane is suitable.1
4.5 Proportoinal control of a CSTBR with
Monod's kinetics
Before designing the closed - loop continuous bioreactor, one should understand the open - loop CSTBR fully since the scope of closed - loop CSTBR will be given only by the knowledge about the open - loop CSTBR. Linear stability analysis and phase plane analysis for open - loop CSTBR and closed - loop CSTBR are detailed below.
4.5.1 Stability analysis of open Ė loop CSTBR
4.5.1.1 Linear stability analysis for open Ė loop CSTBR
As already discussed, for an open - loop CSTBR with Monodís kinetics, there exist two steady states, i.e., a nontrivial steady state (defined by equation (8), (9), and (10)), and washout steady state (defined by equation (11) and (12)). The Jacobian J for the system defined by equation (4), and (7) with m given by equation (2) is
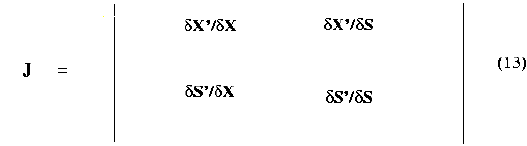
where X' = dX/dt
S' = dS/dt
Substituting the values for Xí and Sí from equations (4) and (7) yields the value of Jacobian for the system as
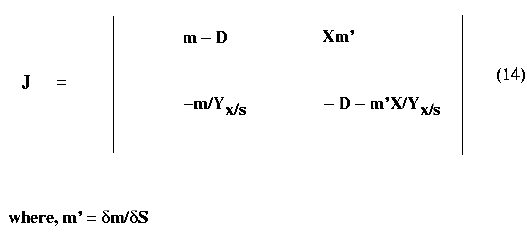
For the nontrivial steady state, stability is guaranteed if the following equations are satisfied
Trace J < 0 (15)
Det J > 0 (16)
Which yields
-D - m'X/ Yx/s < 0
(!7)
Xm'm/ Yx/s > 0 (18)
In case of Monod's equation, m' > 0 for all S. Hence, nontrivial state is always stable. For the washout state, the conditions for stability derived from a similar procedure are
m (Sf) - D < 0
(19)
(m (Sf) - D)(-D) > 0 (20)
Equations (19) and (20) indicate that D must be greater than m (Sf) for the washout steady state to be stable. Thus, any dilution rate which gives any realistic solution (X > 0, and S > 0) will result in washout being unstable. The same conclusion can be derived by the phase plane analysis also which is discussed in the next subsection.
4.5.1.2 Phase plane analysis for open - loop CSTBR
Construction of the phase plane for open - loop CSTBR be achieved via integrating equations (5) and (8), selecting several time points, plotting the values of S and X at each point, then repeating for new initial conditions or sketched directly from the results of the linear analysis. As shown in Figure 3, all initial conditions result in achievement of the desired steady state.1
The motive for controlling this reactor would be to maintain a closed - loop system such that washout could be avoided regardless of flow fluctuations. An easy approach to achieve this would be to measure the cell concentration and manipulate the flow rate to force the reactor to nontrivial steady state. This can
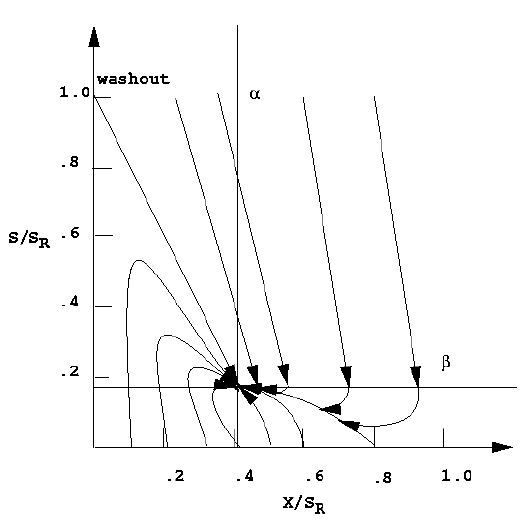
Figure 3. Open loop phase plane for bioreactor with Monodís kinetics (SR is the feed substrate concentration and alpha and beta are the steady state cell and substrate concentrations)
easily be accomplished with a simple proportional controller whose stability analysis is discussed in next subsection.
4.5.2 Stability analysis of closed - loop bioreactor
The governing equation for the proportional controller which manipulates the flow rate as a response to changing cell concentration inside the reactor is given by
D = Dss + Kc(X - Xsp) (21)
Where, D = dilution rate that is manipulated by the controller
(hr-1)
Dss = dilution rate corresponding to the nontrivial
steady state for X = Xsp in open loop CSTBR (hr-1)
Kc = controller gain (l/g-hr)
X = cell concentration in the reactor (g/l)
Xsp = controller set point and the desired cell
concentration in the reactor (g/l)
Substituting the value of D from equation (21) into equations (4) and (7) shows that X = 0, S = Sf is no longer a steady state solution. A rigorous analysis for this system will show that the worst case for this system as X approaches 0 corresponds to D = 0. This is equivalent to saying that the CSTBR will approach the behavior of a batch reactor.
The conditions for the stability of the system under consideration according to linear stability analysis are
-Kc - Dss - m'/ Yx/s < 0
(22)
Kc + m'/ Yx/s > 0
(23)
Any positive value of Kc is sufficient to satisfy equations (22) and (23), and hence guarantee stability. This is not surprising keeping in mind the stability of nontrivial steady state in open - loop CSTBR. It seems fair to expect the closed - loop phase plane similar to open - loop phase plane for reasonable values of Kc.
The whole discussion can be summarized as follows. Since the nontrivial state is always stable for realistic D values, there is little incentive for closed - loop operation other than to prevent washout from large flow disturbances. The incentive for closed - loop operation increases significantly if the growth kinetics are more complex, e.g., substrate inhibited growth kinetics. This is discussed in the next section.
4.6 Control of a continuous bioreactor with substrate inhibition kinetics
Though Monod's kinetics makes a nice model for substrate
- limited cases, it does not approximate the real cases very well
since all the biological systems are inhibited by high substrate
concentration. Hence, understanding these kind of reactors are important.
The dynamics of the CSTBR with substrate inhibition kinetics leads to an
interesting control problem. Interested reader may find detailed analysis
by Dibiasio for an open - loop as well as closed - loop CSTBR with
substrate.1
A number of reports have been published regarding control
issues related to continuous bioreactors. It is suggested to read the given
references 6 - 9 to know more about the subject.
4.7 Fed batch reactor dynamics
Though CSTBR is an excellent tool to study bacterial metabolism, it is not extensively used in biotechnology - industry. The most widely used fermentation mode for industrial production of biochemicals is fed - batch fermentation. The fed - batch system is an interesting system to study since it does not have a true steady state. In this case, evaluation of the state variables will locate the position of the system on a trajectory through the operational cycle. Since these state variables can not be measured online, the estimation of state becomes an important element of optimization and control of the reactor. There are numerous reports about the theoretical and experimental issues related to the fed - batch fermentation. To begin with, the reader is recommended to go through the references 10 - 14.
A convenient method of classifying the various types of possible dynamic behaviors that can be exhibited by a fermentation model is provided by bifurcation analysis. This theory has a large literature, and the interested reader is directed to Razon and Schmitz.15
| BACK | MAIN | NEXT |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Ref. |