|
|
Biology of Methanogenesis |
Department of Marine Biotechnology UMBC - Institute of Marine & Environmental Technology
Kinetics and Threshold Level of PCB Halorespiration
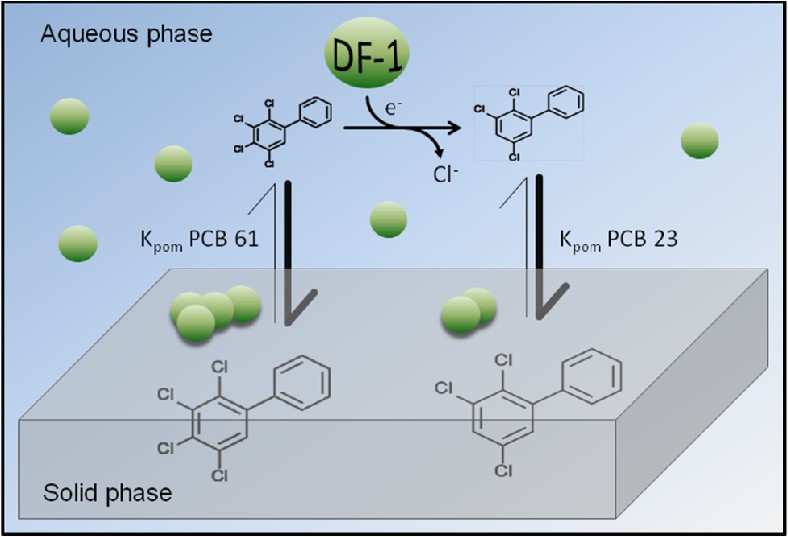
The time required for a PCB-contaminated site to recover cannot yet be predicted due in part to lack of quantitative information on rates of PCB dechlorination in porewater phase. We developed a method to measure rate of dechlorination in the aqueous phase at very low PCB concentrations. This approach utilizes a polymer functioning concurrently as a passive dosing system for maintaining a steady state PCB substrate concentration in the water phase and as a passive equilibrium sampler to monitor the dechlorination product. Rates of dechlorination of 2,3,4,5-tetrachlorobiphenyl (PCB 61) to 2,3,5-trichlorobiphenyl (PCB 23) by an organohalide respiring bacterium, Dehalobium chlorocoercia DF-1, were measured over an environmentally relevant range of 1 ng L-1 to 500 ng L-1 in sediment-free medium using a high concentration of cells (>106 cells mL-1). The results indicate that rate of dechlorination is a linear function of PCB substrate concentration below the maximum aqueous solubility of PCB 61 and occurs at concentrations as low as 1 ng L-1. Demonstration of PCB 61 dechlorination at environmentally relevant concentrations suggests that low numbers of organohalide respiring bacteria rather than bioavailability accounts for low rates of dechlorination typically observed in sediments. Using passive samplers to measure the concentration of dissolved PCBs in the porewater combined with knowledge of congener-specific rates for organohalide respirer(s), it will be possible to project the in situ rate and final concentration of PCBs for a specific site after treatment by bioaugmentation.
 Collaborators
Collaborators
Hal D. May, Ph.D., Medical University of South Carolina
Upal Ghosh, Ph.D., University of Maryland Baltimore County
 Project Team
Project Team
Nathalie Lombard
Rayford Payne, Ph.D.
 Related Publications and Abstracts
Related Publications and Abstracts
Lombard, Nathalie J., Upal
Ghosh, Birthe V. Kjellerup, Kevin R. Sowers. 2014. Kinetics
and threshold level of 2,3,4,5-tetrachlorobiphenyl dechlorination by an
organohalide respiring bacterium. Env. Sci. Technol.
48 (8), pp 4353–4360
Funded by