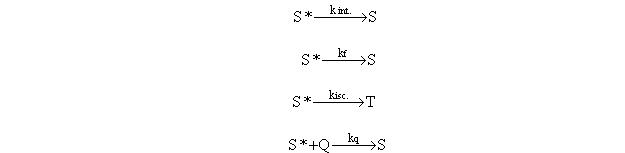Two
sets of data are presented for data analysis.
The first set is the vapor pressure of three substances at various
temperatures. Clausius-Clapeyron
theory relates the vapor pressure of a pure substance to temperature and
quantities such as the heat of vaporization and the heat capacity.
This theory provides an interpretation of the parameters derived by
fitting to the appropriate mathematical function of the vapor pressure as it
varies with temperature according to theory.
The
next set of experimental data is a fluorescence decay curve obtained by the
single-photon-counting method. In
this case, similar to the example given in SGN, the need for appropriate
weighting factors applied to the data will be demonstrated.
In
both analyses, a mathematical expression is fit to the experimental data
using the criterion of least squares as a measure of the goodness fit.
That is, the sum of the squares of the differences of the
experimental points and the mathematically predicted values is minimized
with respect to a set of adjustable parameters.
Theory attempts to justify the mathematical expression that is fitted
to the data and allows for an interpretation of the parameters derived
during the fitting process.
PART I
The
Clausius Clapeyron equation may be derived by considering the Gibbs free
energy of a pure substance between two phases at equilibrium.
The equilibrium condition between two phases a,b is Ga(T,
P) = Gb(T, P). If a temperature or
pressure change perturbs the system, the condition for a new equilibrium is
![]()
![]()
The equation rearranges to the Clapyron eq.:
![]()
![]()
At equilibrium,
![]()
and, substituting in for DS from the above eq.:
![]()
For
liquid to gas (vaporization) or solid to gas (sublimation) phase changes a
simplification can be used. Since the molar volume of the condensed phase is
much smaller than the molar volume of the gas, the molar volume of the solid
(or liquid) may be ignored. Now
molar volume of the gas closely approximates the volume change during the
phase transition. Assuming
ideal gas behavior we obtain the following relations.
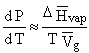
and, for an ideal gas:
![]()
![]()
using: DP/P = d(ln P). Using the above assumptions, the exact Clapyron eq. has been turned into the approximate Clausius-Clapyron equation.
If
DHvap
is constant over a range of
temperatures then we can directly integrate this relation to obtain equation
1.
(1)
![]()
However, if DHvap
is not constant, but instead has a
temperature dependence:
![]()
Substituting this
into the expression above leads to
![]()
![]()
Integration now
yields equation 2.
(2)
![]()
The
parameters K1 and K2 in the above equation allow for
the inclusion of small changes in
![]() as a function of temperature.
as a function of temperature.
To
Do:
(1)
Assume
initially that
![]() does not change with temperature. Choose three substances out of the five listed below and fit,
using a linear regression analysis, the vapor pressure data to
obtain
DHvap
according to equation 1.
Construct a plot and the residuals as described elsewhere in this
module. After reviewing a plot of the residuals (which you will put into your
report) discuss whether the more detailed series in T using equation 2 (see
below) is
necessary to fit to the data accurately.
does not change with temperature. Choose three substances out of the five listed below and fit,
using a linear regression analysis, the vapor pressure data to
obtain
DHvap
according to equation 1.
Construct a plot and the residuals as described elsewhere in this
module. After reviewing a plot of the residuals (which you will put into your
report) discuss whether the more detailed series in T using equation 2 (see
below) is
necessary to fit to the data accurately.
(2) Refit your
data to the non-linear temperature-dependent DHvap
equation (eq. 2). Do this in two ways: (a) using a multiple
linear least squares analysis as described in SG&N and (b) using the Solver
capabilities of Excel. In both cases, construct an x-y plot, overlay
your fits, and (on the same graph) display your residuals. Compare and
contrast the results from each fitting process. As described in SG&N,
discuss the merits of fitting to the more complex equation (fitting to eq. 1
vs. eq. 2). Consider both the statistical arguments as well as
inspection of your residuals. Present in your report a tabulated summary of the
thermodynamic parameters you calculated from each of the analyses. Include a detailed discussion of the criteria you used to
warrant or discount the need for using the more complex equation 2.
If you find that a multiple linear regression is warranted, be sure
that you use the correct coefficients that correspond to each term. Your
analysis should include a discussion of the correlation coefficient, simple
residuals, and F-statistics that are used for determining “goodness of
fit.” You should also compare
and contrast the uncertainties in the parameters obtained from each
analysis. You must demonstrate
that you show a working “understanding” (i.e. don’t just repeat the
definitions given in the book, but discuss, in your own words) of what each
of these statistical quantities mean.
TABLE I: Vapor Pressure Data
Vapor pressures (mm Hg) of less than one atmosphere as a function of
temperature. (All temperatures are in degrees Celsius)
| Chemical | 1 | 5 | 10 | 20 | 40 | 60 | 100 | 200 | 400 | 760 mm Hg |
| Sodium | 439 | 511 | 549 | 589 | 633 | 662 | 701 | 758 | 823 | 892 oC |
| 1,4-Dioxane | -35.8 | -12.8 | -1.2 | 12.0 | 25.2 | 33.8 | 45.1 | 62.3 | 81.8 | 101.1oC |
| Acetone | -59.4 | -40.5 | -31.1 | -20.8 | -9.4 | -2.0 | 7.7 | 22.7 | 39.5 | 56.5 oC |
| Butyric Acid | 25.5 | 49.8 | 61.5 | 74.0 | 88.0 | 96.5 | 108.0 | 125.5 | 144.5 | 163.5 oC |
| Stannic Chloride | -22.7 | -1.0 | 10.0 | 22.0 | 35.2 | 43.5 | 54.7 | 72.0 | 92.1 | 113.0 oC |