


 , Purnima Patel
, Purnima Patel ,
Shamantha Gunawardena
,
Shamantha Gunawardena ,
Jan Powell
,
Jan Powell , Aaron Joseph
, Aaron Joseph , Judith A. Johnson
, Judith A. Johnson and J. Glenn
Morris
and J. Glenn
Morris
1. Department of Chemistry & Biochemistry,
University of Maryland Baltimore County, Baltimore, MD 21228.
2. Departments of Medicine and Pathology, University of Maryland School of Medicine and Veterans Affairs Medical Center, Baltimore, MD 21202.
Research supported by NSF Grant MCB 91-05586 to C.A.B. and by grants from the Department of Veterans Affairs and the National Oceanic and Atmospheric Administration (grant NA36FD0224) to J.G.M
Running title: Analysis of Vibrio vulnificus Polysaccharides
* Corresponding author -- ph. (410) 455-2506, fax (410) 455-2608, e-mail bush@umbc.edu
Abstract Pathogenic bacteria are often classified on the basis of the complex polysaccharides found on the surface, usually capsular polysaccharides or lipopolysaccharides. It is common in clinical practice to use reactivity with antisera specific to the various cell surface carbohydrates for this purpose. In this work, we describe a chemotyping method for bacterial capsular polysaccharides which is based on a carbohydrate analysis of an acid hydrolysate of the capsule. High performance anion exchange chromatography at high pH (HPAE) with electrochemical detection, which is used for analysis of the hydrolysate, shows preferential sensitivity for sugars. A single acid hydrolysis condition is chosen for screening a large collection of bacterial isolates and a computerized autosampler is used to make possible a large number of rapid analyses. This procedure does not yield a quantitative carbohydrate analysis for the sample but produces a fingerprint which can be used to discriminate among isolates which have different capsular polysaccharide structures. The procedure has been applied to a collection of 120 isolates of Vibrio vulnificus, a water born species common in shellfish which causes septicemia in immunocompromised individuals, most often from eating of raw oysters. The collection of bacterial isolates includes strains from both clinical cases of septicemia and from such environmental sources such as sea water, sediments and shellfish. Our results show that a number of unusual sugars including many amino sugars are found in these polysaccharides and that a wide variety of capsular carbotypes in V. vulnificus may be readily distinguished by the HPAE fingerprint.
1. Introduction
Vibrio vulnificus is a Gram-negative bacterium common to estuarine environments, constituting approximately 8% of total culturable heterotrophic bacteria in the Chesapeake Bay during warmer months. It has also been isolated from virtually 100% of oysters harvested during these same time periods(1). In humans, V. vulnificus has been implicated as a cause of gastroenteritis, wound infections (in wounds exposed to water containing the organism), and a syndrome of primary septicemia (2-5). The primary septicemia is associated with eating raw oysters and occurs almost exclusively among persons who are alcoholic, have underlying liver disease or who are immunosuppressed. Mortality rates for persons with primary septicemia exceed 50%, with patients developing intractable shock and multi-organ system failure despite aggressive medical care and antimicrobial therapy.
V. vulnificus produces a capsular polysaccharide which is essential for
virulence (6). This capsule provides the bacterium
with resistance to serum bactericidal activity and phagocytosis; the
capsular material has also been shown to directly stimulate the
release of TNF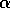
 and other cytokines from peripheral blood mononuclear
cells (7) . Capsular polysaccharide-protein conjugate
vaccines provide protection against lethal infection in mouse models
(8) . Antibodies raised to the purified capsular material
are also protective, both
prior to and after challenge with a fully virulent
V. vulnificus strain. However, protection is provided only against strains
of the homologous capsular type (9) . These observations
underscore the need to carefully catalog
V. vulnificus capsular types, both for studies of the role of the capsular material in
pathogenesis and in exploring development of antisera for possible
therapeutic use.
and other cytokines from peripheral blood mononuclear
cells (7) . Capsular polysaccharide-protein conjugate
vaccines provide protection against lethal infection in mouse models
(8) . Antibodies raised to the purified capsular material
are also protective, both
prior to and after challenge with a fully virulent
V. vulnificus strain. However, protection is provided only against strains
of the homologous capsular type (9) . These observations
underscore the need to carefully catalog
V. vulnificus capsular types, both for studies of the role of the capsular material in
pathogenesis and in exploring development of antisera for possible
therapeutic use.
We have previously reported the isolation, purification, and structural characterization of the capsular polysaccharides from V. vulnificus strains MO6-24 and BO62316 (10-11). We have extended these studies to structure determination for two more clinical strains, 6353 and ATCC 27562, (Reddy and Gunawardena, unpublished results). These structure determinations were carried out largely by high-resolution heteronuclear NMR spectroscopy using methods which are now well established (12) . These results show that there is not a single type of V. vulnificus capsule and suggest that there may be many types.
Chemical analysis of bacterial polysaccharides has been used to establish a chemotype based on carbohydrate composition of the cell surface polysaccharides. Iguchi et al. (13) have used a gas chromatographic method of carbohydrate analysis to correlate composition of the lipopolysaccharide O-antigen sugar chain with the serologically determined types for the related species, V. fluvialis. We have used a similar approach for classification of V. vulnificus capsular types based on an HPLC method. Preliminary results of carbohydrate analysis of the capsular polysaccharide of clinical and environmental isolates of V. vulnificus were used to define 15 different carbotypes based on the presence or absence of certain sugars (14) . The method of carbohydrate analysis which was used in these experiments, HPAE chromatography with electrochemical detection, has the advantage that it requires minimal chemical workup and is amenable to automation by a computer controlled autosampler. Such technology makes it possible to undertake a wide ranging survey which includes a large collection of V. vulnificus strains.
In the present study we describe a protocol for qualitative carbohydrate analysis for V. vulnificus capsules which is suitable for screening of a large collection of strains. The method uses HPAE with electrochemical detection and computer automation. We describe the application of the new method to a large collection of V. vulnificus strains (Powell et al., in preparation). We show that there is a wide variety of carbohydrate composition in V. vulnificus capsules and that they are rich in amino sugars, as has been found for the cell surface polysaccharides of other species of vibrio. While the method described is clearly for research purposes rather than clinical use, it is possible that a simplified small scale capsule isolation could be developed for use with a microbore HPAE or capillary electrophoresis assay.
II. Materials and Methods
The clinical strains of V. vulnificus which are the subject of this study have been described previously (14) and additional environmental strains were collected by Tamplin and coworkers, (15) . The collection has also been extensively characterized by other data including ribotyping which will be reported elsewhere (Powell et al, in preparation).
Single bacterial colonies grown from frozen glycerol stocks were inoculated
into L-broth for 18 hrs growth at 30 C . 1 mL of each culture was spread
on 350 cm
C . 1 mL of each culture was spread
on 350 cm of L-agar and grown 18 hrs at 30
of L-agar and grown 18 hrs at 30 C . Bacterial lawns were then
scraped off with a glass slide into 30 mL oakridge tubes, 15 mL of 50%
PBS was added, and the tubes were shaken at 200 rpm for 1 hr at 25
C . Bacterial lawns were then
scraped off with a glass slide into 30 mL oakridge tubes, 15 mL of 50%
PBS was added, and the tubes were shaken at 200 rpm for 1 hr at 25 C .
The bacteria were pelleted and the supernatant wash was poured into 100Kda
molecular weight cutoff Centriprep unit (Amicon, Beverly, MA) and spun at
500 x g for 1 hr. DNAase, RNAase and Pronase E (Sigma, St. Louis, MO) were
sequentially added at 2 hr intervals to each of the retentates and
incubated at 37
C .
The bacteria were pelleted and the supernatant wash was poured into 100Kda
molecular weight cutoff Centriprep unit (Amicon, Beverly, MA) and spun at
500 x g for 1 hr. DNAase, RNAase and Pronase E (Sigma, St. Louis, MO) were
sequentially added at 2 hr intervals to each of the retentates and
incubated at 37 C . After enzyme treatment the retentates were spun
and washed with two volumes of endotoxin free water. Finally the retentate was
ultracentrifuged at 100,000 x g for 18 hrs, the supernatant was extracted
sequentially with phenol then chloroform two times and the aqueous phase was
lyophilized and stored at -20
C . After enzyme treatment the retentates were spun
and washed with two volumes of endotoxin free water. Finally the retentate was
ultracentrifuged at 100,000 x g for 18 hrs, the supernatant was extracted
sequentially with phenol then chloroform two times and the aqueous phase was
lyophilized and stored at -20 C for carbohydrate analysis.
C for carbohydrate analysis.
For carbohydrate analysis, the polysaccharide sample was dissolved
in
water at a concentration of 1 mg/ml. A 200 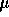 L aliquot was removed
and
dried under a stream of N
L aliquot was removed
and
dried under a stream of N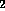 . Aqueous acid (either HCl or trifluoroacetic acid)
was added and the sample was incubated in a capped tube in a heating block
for fixed time intervals of 2 to 17 hrs. After hydrolysis, the acid was
evaporated
under a stream of N
. Aqueous acid (either HCl or trifluoroacetic acid)
was added and the sample was incubated in a capped tube in a heating block
for fixed time intervals of 2 to 17 hrs. After hydrolysis, the acid was
evaporated
under a stream of N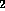 and the residue dissolved in 200
and the residue dissolved in 200 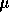 L of
deionized
water. HPLC was carried out on a Dionex Glycostation with autosampler. 20
L of
deionized
water. HPLC was carried out on a Dionex Glycostation with autosampler. 20
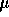 L
of sample was injected to a CarboPAC PA-1 column which was eluted with two
different protocols, one for acidic sugars and a second for neutral and amino
sugars ( 16) . For analysis of acidic sugars, such as
uronic
acids, elution began with 100 mM NaOH plus 150 mM NaOAc for 30 minutes.
The
analysis was followed by a rinse with 400 mM NaOAC and 100 mM NaOH for 15
minutes.
Then the column was equilibrated with the running solvent for 15 minutes
before a new sample was injected.
L
of sample was injected to a CarboPAC PA-1 column which was eluted with two
different protocols, one for acidic sugars and a second for neutral and amino
sugars ( 16) . For analysis of acidic sugars, such as
uronic
acids, elution began with 100 mM NaOH plus 150 mM NaOAc for 30 minutes.
The
analysis was followed by a rinse with 400 mM NaOAC and 100 mM NaOH for 15
minutes.
Then the column was equilibrated with the running solvent for 15 minutes
before a new sample was injected.
For detection of sugars which lack a formal anionic charge, elution was by 16 mM NaOH for 25 minutes followed by a wash with 50 mM NaOH plus 200 mM NaOAc for 12 minutes. The column was then reequilibrated with running solvent for 23 minutes. This method is suitable for neutral sugars and amino sugars. In both cases the detection was by pulsed amperometric detection using a gold working electrode. The following pulse potentials and durations were used: E1=0.05volts(t1=300ms); E2=0.65volts(t2=180ms); E3=-0.65volts(t3=60ms). An IBM PC interfaced with Dionex AI-450 software was used for data collection and instrument control.
For identification of HPLC peaks detected by PAD, three different methods were used. For most definitive identification of sugars with HPLC peaks, chromatograms were inspected and tentative identifications of sugars were made on the basis of comparison of retention times with standards. Then authentic samples of monosaccharides were co-injected with the hydrolysate. Chromatographic identity of a sugar was confirmed if the co-injection caused no change in the peak shape on visual inspection. This lengthy procedure, which requires that authentic samples be available for all sugars in the sample, is needed for positive identification. The method of peak authentication by co-injection has been used for the chromatograms of polysaccharide hydrolysates of the strains of V. vulnificus reported in the paper of Hayat et al ( 14) . This method requires a number of HPLC runs for each polysaccharide sample for positive identification of a sugar. For four extensively studied strains of V. vulnificus , namely MO6-24, BO6-2316, 6353 and ATCC 27562, the identities of the sugars was further confirmed by NMR spectroscopy (10-11 and unpublished results. )
A second, less time-consuming method for identification which involves co-injection of a single internal standard with the sample was also used in this study. Internal standards were co-injected with the sample allowing relative retention times to be calculated and allowing for the inclusion of unknown sugars in a fingerprint characteristic of a capsular polysaccharide. An important advantage of the second method is that it does not require authentic standards for sugars and thus can be used for HPLC peaks for which no known sugar can be assigned. This method requires two chromatograms for each analysis, one with and one without the internal standard, fucose and mannose. This method was used for the neutral sugar analysis (16 mM NaOH protocol) of 33 of the samples reported in this study.
A third method was used for peak identification for all the acidic sugar runs and for the remainder of the 16 mM NaOH runs. In this case external standard sugars were run at the start of each autosampler run and again at the end for the purpose of calculating relative retention times. Typically each autosampler run was 8 to 10 hours long with 6 to 8 polysaccharide samples in between the standard runs. Although the precision of identification was slightly reduced in this method when compared to the internal standard method, the reproducibility of the retention times is adequate for the purposes of this study. Reproducibility is substantially improved by the use of the autosampler program with the computer programmed wash and equilibration steps. This improvement was especially important for the 16 mM NaOH protocol.
Although amino compounds and sulfur-containing compounds can be detected at lower sensitivity in some cases, the electrochemical method of detection in HPLC is generally quite selective for sugars and other polyols, (17). Therefore, most of the HPLC peaks are expected to represent carbohydrate components of the capsular polysaccharide.
III. Results and Discussion
Hydrolysis of the polysaccharides.
Acid hydrolysis of a complex polysaccharide to produce a quantitative yield of all the constituent monosaccharides is notoriously difficult and it is not generally possible to cleave quantitatively a bacterial polysaccharide into its component sugars without partial degradation of some of the sugars. This problem is serious in a system whose carbohydrate composition is unknown since some sugar linkages may be especially resistant to hydrolysis and some monosaccharides are especially susceptible to acid degradation. Both capsules and lipopolysaccharides of the genus Vibrio are rich in amino sugars and uronic acids both of which are very resistant to hydrolysis. Moreover these polysaccharides also contain deoxy and di-deoxy sugars which are especially labile to acid degradation. These features make it difficult to devise a single hydrolysis protocol for quantitative carbohydrate analysis in a large collection of strains which may differ widely in composition in ways not known in advance.
Therefore we sought hydrolysis conditions to optimize the information content of each chromatogram. Hydrolysis conditions were tested on capsular polysaccharides from four clinical strains of V. vulnificus , each with four sugar residues in the repeating subunit. MO6-24 has three residues of quiNAc and one residue of galNAcA (10). BO6-2316 has one residue each of quiNAc, rhaNAc, fucNAc and galNAcA (11) . Strain 6353 has one residue each of quiNAc, galNAc, galNAcA and glcNAcA while ATCC 27562 has one residue each of glcNAc, rha, muramic acid and galA (Reddy and Gunawardena, unpublished results). Preliminary analysis of the carbohydrate composition of these four capsules with 1 M and 2 M TFA was evaluated. This standard method is commonly used for carbohydrate analysis of glycoproteins as well as other other complex polysaccharides, ( 16) . The results of this preliminary study were not very satisfactory giving poor yields of monosaccharides for the four polysaccharides of known structure (data not shown). Results were improved with more vigorous hydrolytic conditions using HCl in concentrations ranging from 2 M to 6 M and times ranging from 1 hr to 17 hr. The improved results with stronger hydrolytic conditions may result from high content of these polysaccharides in amino sugars which tend to be relatively stable in strong acid.
In Fig. 1 we show the neutral and acidic sugar analyses for hydrolysates of
the capsular polysaccharides of the four type strains
under conditions of 4 M HCl for 4 hr at 100 C . Each of these strains
contains
four sugars in the repeating subunit and for all cases, each of the component
sugars gave an HPLC peak. There is not a simple correspondence of peak
heights to carbohydrate composition in these samples for several reasons.
First, the electrochemical response of sugars varies somewhat among different
monosaccharides and molar response factors differ for different
sugars ( 18) .
In principle this problem could be overcome by use of authentic standards for
peak heights of
each of the known sugars. Unfortunately this can be difficult for rare sugars
which are not routinely available in crystalline form. Such sugars are common
in the V. vulnificus polysaccharides. But a second problem which is not so easily
overcome is the failure to cleave completely all the glycosidic linkages,
especially those of the uronic
acids. It is known that one of the peaks in the chromatographic analysis of
the acidic sugars in the polysaccharides from strains MO6-24
and BO6-2316 is a disaccharide whose linkage is quite resistant to hydrolysis,
galNAcA
C . Each of these strains
contains
four sugars in the repeating subunit and for all cases, each of the component
sugars gave an HPLC peak. There is not a simple correspondence of peak
heights to carbohydrate composition in these samples for several reasons.
First, the electrochemical response of sugars varies somewhat among different
monosaccharides and molar response factors differ for different
sugars ( 18) .
In principle this problem could be overcome by use of authentic standards for
peak heights of
each of the known sugars. Unfortunately this can be difficult for rare sugars
which are not routinely available in crystalline form. Such sugars are common
in the V. vulnificus polysaccharides. But a second problem which is not so easily
overcome is the failure to cleave completely all the glycosidic linkages,
especially those of the uronic
acids. It is known that one of the peaks in the chromatographic analysis of
the acidic sugars in the polysaccharides from strains MO6-24
and BO6-2316 is a disaccharide whose linkage is quite resistant to hydrolysis,
galNAcA 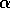 -
- 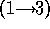 - quiNAc, (10,19). For hydrolysis in 2N to 6N HCl for times up
to 17 hours, this dimer peak persists. In fact the chromatographic patterns
(data not shown) for most of these hydrolyses do
not differ greatly from those of Fig. 1 and under no conditions could the
the disaccharide be cleaved. Under conditions as strong as 6N HCl for 17 hrs it
is very likely that there is considerable loss of sugars such as galactose and
rhamnose.
- quiNAc, (10,19). For hydrolysis in 2N to 6N HCl for times up
to 17 hours, this dimer peak persists. In fact the chromatographic patterns
(data not shown) for most of these hydrolyses do
not differ greatly from those of Fig. 1 and under no conditions could the
the disaccharide be cleaved. Under conditions as strong as 6N HCl for 17 hrs it
is very likely that there is considerable loss of sugars such as galactose and
rhamnose.
Therefore 4N HCl for 4 hr was selected as a standard condition for generating a fingerprint for the polysaccharides of this group. We recognize that not all the linkages are cleaved quantitatively and that some of the more labile sugars may be partially degraded. But we propose that these conditions generate a fingerprint chromatogram characteristic of different polysaccharides. Although not all the sugars can be identified, HPLC peaks corresponding to unknown sugars can be specified and included in the fingerprint and their presence or absence can be used for defining a capsular carbotype.
Peaks in the
chromatogram were characterized by retention time and assigned if possible to
a specific monosaccharide. The presence or absence of peaks could then be
used to characterize differing types of capsular polysaccharide structure.
For the identification of peaks with particular sugars,
retention times were compared with standards.
The polysaccharides of 30 different
strains of V. vulnificus were hydrolyzed under the standard
conditions, ( 4 N HCl, 4 hr, 100  C ). HPLC analysis for each
polysaccharide hydrolysate in 16 mM NaOH was carried out with the
autosampler and in the run which immediately followed,
fucose and mannose were co-injected with the same polysaccharide as
internal standards. From these chromatograms, several conclusions were evident.
First, the autosampler protocol gave HPLC peak
retention times which were reproducible to
C ). HPLC analysis for each
polysaccharide hydrolysate in 16 mM NaOH was carried out with the
autosampler and in the run which immediately followed,
fucose and mannose were co-injected with the same polysaccharide as
internal standards. From these chromatograms, several conclusions were evident.
First, the autosampler protocol gave HPLC peak
retention times which were reproducible to  0.1 min on a single day and
to
0.1 min on a single day and
to  0.3 min for runs on the same column a month later. Under these
conditions, the use of retention times relative to a standard run on the
same day can be expected
to give a retention times accurate to about
0.3 min for runs on the same column a month later. Under these
conditions, the use of retention times relative to a standard run on the
same day can be expected
to give a retention times accurate to about  0.2 min. For the stronger elution
conditions used for detection of acidic sugars, reproducibility is somewhat
better being about
0.2 min. For the stronger elution
conditions used for detection of acidic sugars, reproducibility is somewhat
better being about  0.1 min. A second finding was that there is a wide
variety of sugar compositions in this group of 30 strains. Therefore it is not
difficult to distinguish a large number of different capsular types in this
system even
without highly precise chromatographic analysis. The patterns of HPLC peaks
are quite different among different strains of V. vulnificus
and we can easily distinguish many different types. While
most of the HPLC peaks could be identified with sugars, some peaks remain
unknown. However their presence or absence is readily distinguishable
and is useful in distinguishing capsular types.
0.1 min. A second finding was that there is a wide
variety of sugar compositions in this group of 30 strains. Therefore it is not
difficult to distinguish a large number of different capsular types in this
system even
without highly precise chromatographic analysis. The patterns of HPLC peaks
are quite different among different strains of V. vulnificus
and we can easily distinguish many different types. While
most of the HPLC peaks could be identified with sugars, some peaks remain
unknown. However their presence or absence is readily distinguishable
and is useful in distinguishing capsular types.
Chromatograms are shown in Fig. 2a for neutral sugars of several selected
strains of V. vulnificus . Sugar patterns for six different types are shown to illustrate that it
possible to distinguish readily a number of different types. This figure shows
that the neutral sugar
pattern for strains 442 and 444 are somewhat similar
giving mainly fucosamine, fucose and galactosamine. But the patterns for the
acidic sugars of these two strains (Fig. 2b) are readily distinguishable. Strain
444 shows galacturonic acid and a peak at 4.4 mins which is not a known
sugar. Strain 442 shows mainly a single peak at 5.20 mins which has not
been associated with any known sugar. Although the corresponding
chromatogram for strain 435 also has this same sugar, it has not been
observed in other strains. Strain 401 from sediment in Brevard County, FL
shows a pattern very similar to a
previously studied clinical strain 6353 from Maryland ( 14) and it is assigned
the carbotype (type 3) used in that earlier study. The acidic sugar
chromatogram for this strain has a peak at 3.80 min which is very close to the
disaccharide galNAcA 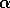 -
- 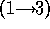 - quiNAc (3.67 min).
These peaks can be distinguished by co-injecting samples but cannot be
distinguished by retention times since difference is close to the experimental
error. The assignment of strain 401 to carbotype 3 has not been confirmed
by NMR spectroscopy or other analytical methods.
- quiNAc (3.67 min).
These peaks can be distinguished by co-injecting samples but cannot be
distinguished by retention times since difference is close to the experimental
error. The assignment of strain 401 to carbotype 3 has not been confirmed
by NMR spectroscopy or other analytical methods.
For chromatographic fingerprints as different as those of Fig. 2, the capsular polysaccharides almost certainly have differing structures. But similar patterns for two polysaccharides do not prove the structures are identical for several reasons. First, similar fingerprints do not prove identical carbohydrate composition since two different sugars can have retention times so similar as not to be distinguishable. Second, the method does not provide rigorous quantitative analysis of the sugars so two strains with similar chromatograms could have different numbers of the constituent sugars in the repeating subunit. Third, even if the carbohydrate composition of the repeating subunit of two polysaccharides is identical, the linkages and anomeric configurations could differ. But Fig. 2 does illustrate the wide variety of patterns allowing discrimination of a number of different capsular types.
Table 1 lists the nominal retention times of the peaks which were used in
distinguishing carbohydrate types in the classification of the V. vulnificus
capsules. In the identification of peaks in the individual chromatograms,
retention times relative to fucose and mannose were used to reduce the effects
of fluctuations and irreproducibility of exact retention times discussed
above. This is especially important for the neutral sugars eluted with 16 mM
NaOH. In the chromatograms for the acidic sugars, galacturonate was used as
external standard. The assignment
of peaks to sugars in this table should be considered tentative. Most were
not tested by co-injection which is generally considered essential for
positive identification of a sugar in HPAE chromatography. In particular we
did not attempt to distinguish between the disaccharide galNAcA 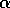 -
- 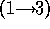 -
quiNAc and the unknown sugar at 3.8 minutes in the
chromatograms. Also muramic acid elutes very close to galactosamine
uronate. The former sugar was unambiguously identified in strain ATCC 27562
by co-injection with authentic standards and by NMR spectroscopy
(S. Gunawardena and G.P. Reddy, unpublished results). But we have not
attempted to distinguish between these two sugars in this screening project
and have arbitrarily assigned the peak to galactosamine uronate in the other
strains. The sugars which are not identified in Table 1 were found not to
coincide with any common sugars or with any we have found in our other studies
of vibrio polysaccharides.
-
quiNAc and the unknown sugar at 3.8 minutes in the
chromatograms. Also muramic acid elutes very close to galactosamine
uronate. The former sugar was unambiguously identified in strain ATCC 27562
by co-injection with authentic standards and by NMR spectroscopy
(S. Gunawardena and G.P. Reddy, unpublished results). But we have not
attempted to distinguish between these two sugars in this screening project
and have arbitrarily assigned the peak to galactosamine uronate in the other
strains. The sugars which are not identified in Table 1 were found not to
coincide with any common sugars or with any we have found in our other studies
of vibrio polysaccharides.
We have analyzed the chromatograms for both neutral and acidic sugars of the 120 strains of V. vulnificus using the data of Table 1 and assigned distinct capsular types on the basis of presence or absence of the peaks indicated there. In Table 2 are listed the strains tested along with indications of the sources of the bacteria (14-15) and the carbotype which has been assigned. The chromatograms are interpreted to give a crude indication of carbohydrate composition which is encoded in an octal number.
To interpret the octal number in terms of carbohydrate composition, imagine
a reference HPLC chromatogram which includes all the
neutral sugars followed by a chromatogram for all the acidic
sugars. The peaks corresponding to each of the 22 sugars identified in the
study
are arranged in order of the elution times as given in Table 1. Then place the
chromatograms for any individual strain over this reference chromatogram,
once again with the chromatogram for the neutral sugars followed by the
one for the acidic sugars.
For each peak which appears in the chromatogram for
particular strain, place a binary 1 and for each peak which does not appear,
place a binary 0. The resulting binary number contains 22 bits, with each
bit representing one of the sugars listed in the order given in Table 1.
We have encoded this binary number in octal form as a compact representation
in Table 2. To derive a crude representation of the chromatogram for any
individual strain listed in Table 2, expand the octal number representing
its composition to binary form. In the resulting binary number,
1 indicates presence of
the sugar from the list in Table 1 in the order given while a 0 indicates
the absence of the sugar. Thus strain MO6-24 (carbotype 1) is assigned the
binary composition number 0,000,010,000,000,100,001,000 indicating that it
shows only three HPLC peaks which are assigned to sugars in
Table 1. Quinovosamine is identified in the
neutral sugar chromatogram and two peaks (disaccharide and galactosamine
uronate) in are observed in the acid sugar chromatogram.
To convert the 22 bit binary number to octal code, bits are arranged
in groups of 3, beginning with the least significant. (The right most
bit represents glcNH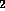 A in Table 1.) Each 3 bit group is represented
by the corresponding octal digit (0-7) and MO6-24 thus is
given the composition number 00200410.
This scheme allows convenient numerical sorting of the strains in
a large collection such as ours, placing related sugar types
adjacent in the carbotype list and ensuring each carbotype is
unique. Two polysaccharides with the same composition
number are assumed to be the same carbotype unless some other
data shows them to be distinct. For the polysaccharides of certain strains,
there are prominent HPLC peaks which are not
included in Table 1 and which are identified as 'Other' in Table 2.
In a few cases, NMR
spectra have also been used to discriminate between different polysaccharide
structures ( 14). Thus
strains MO6-24 and UMCP40a are classified as distinct on the basis of NMR
spectra even though they have
the same composition number.
A in Table 1.) Each 3 bit group is represented
by the corresponding octal digit (0-7) and MO6-24 thus is
given the composition number 00200410.
This scheme allows convenient numerical sorting of the strains in
a large collection such as ours, placing related sugar types
adjacent in the carbotype list and ensuring each carbotype is
unique. Two polysaccharides with the same composition
number are assumed to be the same carbotype unless some other
data shows them to be distinct. For the polysaccharides of certain strains,
there are prominent HPLC peaks which are not
included in Table 1 and which are identified as 'Other' in Table 2.
In a few cases, NMR
spectra have also been used to discriminate between different polysaccharide
structures ( 14). Thus
strains MO6-24 and UMCP40a are classified as distinct on the basis of NMR
spectra even though they have
the same composition number.
There are several reasons why two strains with identical composition numbers, and which are assigned the same carbotype in Table 2, do not necessarily have the same polysaccharide structure. As discussed above, the chromatographic resolution may not distinguish two sugars with similar retention times and the system is not sensitive to the absolute configurations of the sugars. For example, vibrio polysaccharides are known to contains both D and L quiNAc. Even for sugars which are correctly identified as identical, the binary encoding method used in Table 2 indicates only presence or absence of a constituent sugar and not the number of residues in the repeating subunit. Finally, differences in the sequence and linkage of the sugar residues would probably not be distinguished with this simple coding. But even with this crude scheme, 94 different carbotypes can be distinguished among the 120 strains studied. A more detailed analysis could probably further subdivide these carbotypes into an even greater number. The study shows that there must be considerable variety in types of V. vulnificus capsular polysaccharides.
The data of Table 1 show that although the most commonly found sugars are rhamnose and galactose, they are not found in all the samples. Many amino sugars are found including galactosamine, fucosamine, glucosamine and quinovosamine as well as less common amino sugars. A wide variety and abundance of amino sugars has been reported in many studies of the LPS of vibrios, perhaps reflecting the nitrogen-rich marine environment with which they are associated. Most of the strains had peaks in the acidic sugar chromatograms which could be identified as known uronic acids. 63 samples contained galactosamine uronate which is the most common identified uronic acid. A few strains had glucosamine uronate and galacturonate but glucuronate was not found in any sample. Ketodeoxy-octanoate (KDO) had been identified in strain 2809-78 but was not found in any of the newly studied strains in this collection. There are a number of unidentified peaks in the chromatograms of the acidic sugars. The peak at 4.40 minutes was most common. These unidentified peaks could represent rare uronic acids or they could be disaccharides resulting from the failure of our hydrolysis protocol to completely cleave glycosidic linkages of uronic acids.
Conclusions Our results confirm previous studies which indicate that there is a wide variety of both genotypic and phenotypic variation among strains of V. vulnificus . Tamplin et al. ( 15) report an analysis of a large collection of V. vulnificus strains which partially overlaps with the collection upon which we report here. They describe a ribotyping scheme which yields three clusters of strains as well as a pulsed-field-electrophoresis analysis of large DNA fragments which shows a much wider diversity of types. We have developed a ribotyping scheme which differs from that of Tamplin et al.,(15) and which shows a much wider variation among strains of V. vulnificus . None of these genetically based schemes for classification of V. vulnificus shows a strong correlation with the carbotypes listed in Table 2, (Powell et al., in preparation).
Our earlier report on a more limited collection of V. vulnificus strains used antibodies to a protein conjugate along with carbohydrate analysis by HPAE to suggest a variety of capsular types, (14) . Simonsen and Siebling ( 20) also reported on studies of a different group of V. vulnificus strains using antibodies raised to protein conjugated polysaccharides. Their criteria indicated at least three different capsular types within the small collection of strains studied. The results of the present study indicate that there must be a very great diversity of capsular types among V. vulnificus . The data presently available on clinical and environmental strains do not indicate any clear correlation of capsular type with pathogenic potential. No obvious common feature is shared among pathogenic strains isolated from different clinical sources. However it is possible that there could be some particular epitope common to all the clinical strains. Given the limited number of known structures of capsular polysaccharides from clinical and environmental sources, it is not possible to recognize any common structural or conformational feature.
Our results show that in the absence of a simple serotyping system, capsular typing by carbohydrate analysis could provide a practical basis for classification of bacteria such as V. vulnificus . Other chromatographic methods such as GC ( 13) or different HPLC protocols could be used for carbohydrate analysis. Our system could be correlated with a different chromatographic procedure for known sugars for which retention times could be measured to cross reference with our carbotype system. Such a procedure could be used to test the correctness of our identifications of individual sugars since it is unlikely for two different sugars to have the same retention times in two different chromatographic systems. The peaks which were not assigned to any sugar in Table 1 cannot be readily correlated with another chromatographic system. The selectivity of the electrochemical detection method (17) indicates that these peaks are most likely to represent sugars. While these peaks could represent some non-carbohydrate contaminant isolated with the capsular polysaccharide, none of the peaks is common to all strains and most were found in rather few samples.
There are in Table 2 a few instances of several different strains which share the same carbotype. Not surprisingly, there were cases in which several strains collected from the same environmental location all show the same carbotype. Strains 478, 479 and 480 which were all collected from sediment from Bay Adams, LA. are all carbotype 38. But in a few instances, single carbotypes were isolated from several widely dispersed sources. Carbotype 2 has been isolated from clinical cases in MD and NY as well as from an oyster in Delaware Bay, NJ and carbotype 40 was isolated from sediments from Telegraph Reef, MS and from the Yeocomico River, VA as well as from an oyster in Bay Adams, LA. The data of Table 2 suggest that any new collection of environmental strains would be likely to define a number of new and distinct carbotypes according to our procedure. But there would probably be a small overlap of perhaps 10 to 20% with the currently defined types. An additional observation is that strains isolated from a single oyster can have different carbotypes. Strains 128, 129, 132 and 136 all from a single oyster in the Delaware Bay showed carbotypes 73, 49, 76 and 18 respectively. Similarly strains 122, 123 and 124 from a single oyster were carbotype 88, 2 and 65.
ACKNOWLEDGMENT
We thank Dr. M.L. Tamplin for providing a large collection of environmental isolates and Dr. William R. LaCourse for helpful discussions of the chromatographic method.
References
Table 1.
Chromatographic Data on Monosaccharides
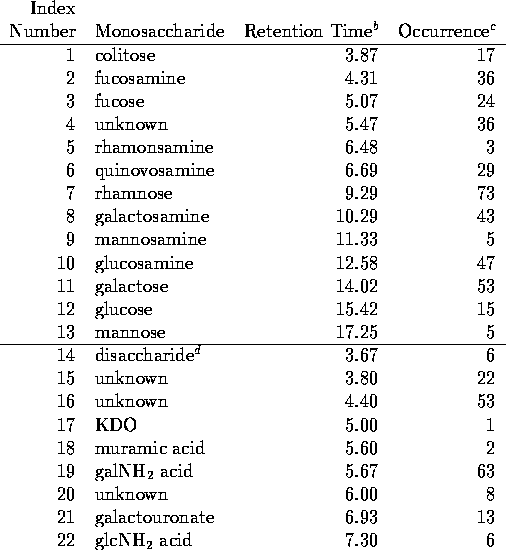
a.) Sugars 1-13 are detected with elution in 16 mM NaOH and sugars 14-22 are detected with elution in 100 mM NaOH + 150 mM sodium acetate.
b.) Time of the void peak, t = 1.65 min.
= 1.65 min.
c.) Occurrence is the number of strains in the list of Table 2 which contain that sugar.
d.) Disaccharide is galNH A
A 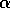 -
- 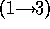 - quiNH
- quiNH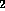 .
.
Table 2
Carbohydrate Compositions of Capsular Polysacchairdes of V. vulnificus
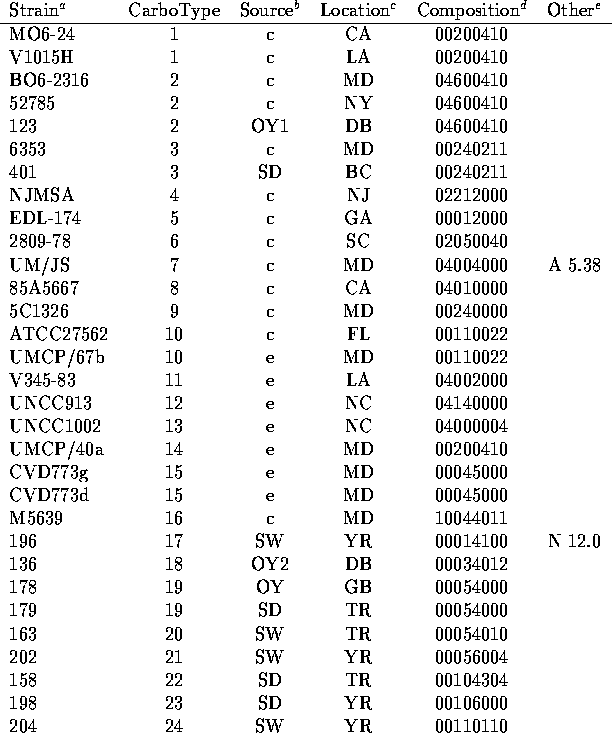
Table II (cont.)
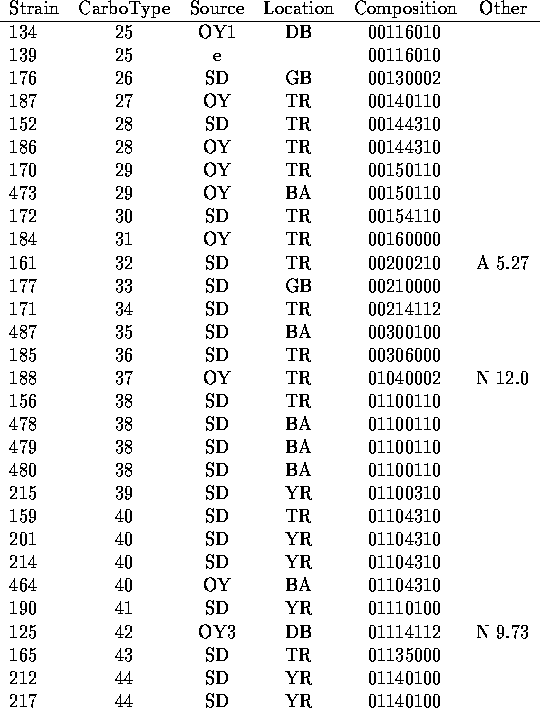
Table II (cont.)
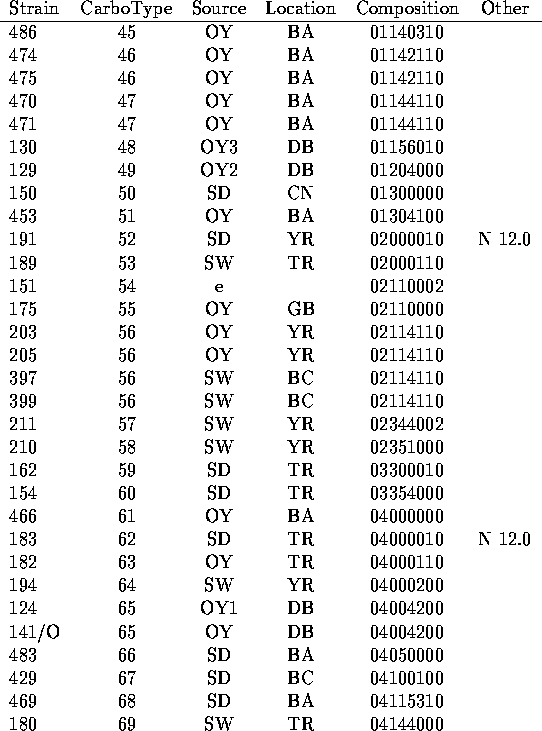
Table II (cont.)
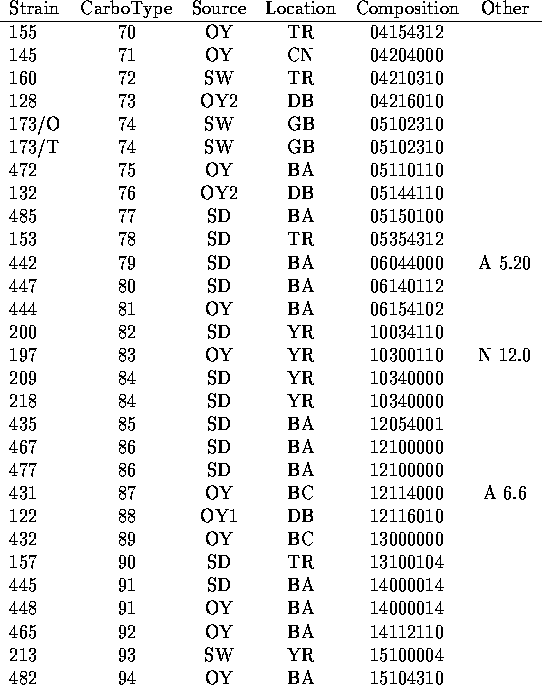
Footnotes:
a.) In cases for which multiple strains are assigned the same carbotype, the first strain listed in Table 2 is the designated type strain.
b.) Source key: OY= oyster (Number following indicates individual oysters.) SD= sediment; SW= seawater; e= other environmental or unknown environmental; c= clinical.
c.) Location key: Standard abbreviations for states plus BA= Bay Adams, LA; BC= Brevard County, FL; CN= Chestnut Neck, NJ; DB= Delaware Bay, NJ; GB= Gaveline Bayou, MS; TR= Telegraph Reef, MS; YR= Yeocomico R.,VA
d.) Octal representation of a binary number which represents presence (1) or absence (0) of constituent sugars listed in the order of Table 1. (See text.)
e.) Prominent HPLC peaks (with time in min) in neutral (N) or acidic (A) sugar analysis which were not found widely in other strains.
Legends for Figures
 C )
of capsular polysaccharides from four strains with known structures having
four sugar residues per repeating subunit.
See Table 1 for retention times. Peaks for MO6-24, neutral quiNH
C )
of capsular polysaccharides from four strains with known structures having
four sugar residues per repeating subunit.
See Table 1 for retention times. Peaks for MO6-24, neutral quiNH ;
acidic: disaccharide, galNH
;
acidic: disaccharide, galNH A. For BO6-2316, neutral: fucNH
A. For BO6-2316, neutral: fucNH , rhaNH
, rhaNH ,
quiNH
,
quiNH ; acidic: disaccharide, galNH
; acidic: disaccharide, galNH A. For 6353, neutral: quiNH
A. For 6353, neutral: quiNH ,
galNH
,
galNH ; acidic: unk 3.8 m, galNH
; acidic: unk 3.8 m, galNH A, glcNH
A, glcNH A. For ATCC 27562,
neutral: rha, glcNH
A. For ATCC 27562,
neutral: rha, glcNH ; acidic: murNH
; acidic: murNH , galA.
, galA.


