Dilution Problem Set
(posted the week of 1/31/00)
I will post the answers to these in a few weeks. In the mean time,
you
can use these to help study for the next quiz. You WILL NOT see
these
problems on upcoming quizzes. They are strictly for practice.
If you can do these, you can do other things that we'll give you.
-
In order to make a solution to isolate DNA from bacteria, a cell biology
student must first make up a 2 M solution of NaOH. The student needs
5 ml and knows that NaOH MW = 40 g/mole. How many grams of NaOH will
the student need?
-
For a later step in isolating DNA, another cell biology student has to
make up a 5 mM solution of NaCl. He weighs out the very last grains
of NaCl and has 10 ug. What volume of water, in ml, does he need
to make his NaCl solution?
-
Design a serial dilution given that you start with 5 M NaCl
and a final concetration of 250 uM.
-
What is the initial concentration in Mole/L?
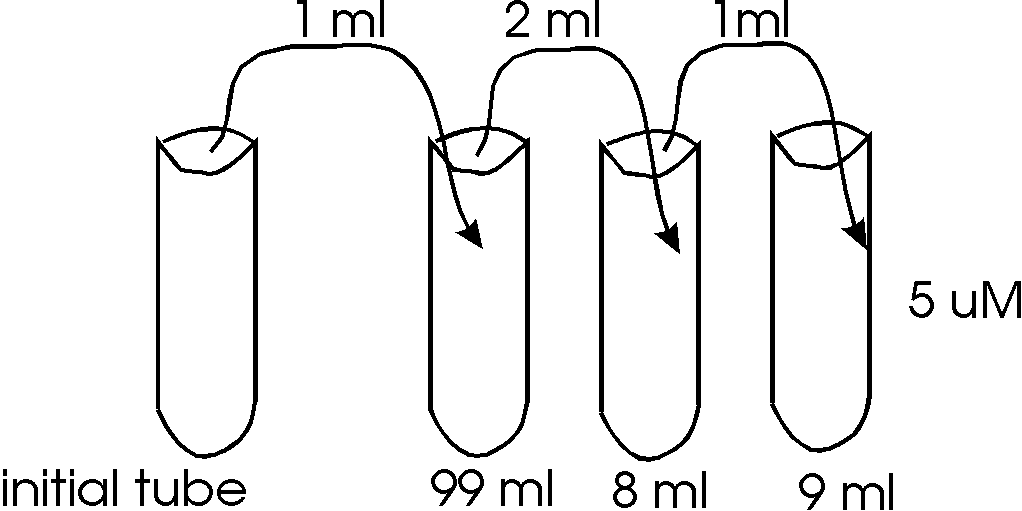
-
A biology grad student has a 1 M solution of a chemical called DTT.
She needs to do a dilution and comes to a cell biology student for help.
Shee wants you tell her what volume of 1 M DTT to use in order to have
100 ul of 100 mM DTT. What do you tell her? Feeling noble,
you also tell her how much diluent (water) to use (the volume in ul).
-
What is the dilution if you put 250 ul of
a solution into a total volume of 5 ml?

