-
In order to make a solution to isolate DNA from bacteria, a cell biology
student must first make up a 2 M solution of NaOH. The student needs
5 ml and knows that NaOH MW = 40 g/mole. How many grams of NaOH will
the student need?
2 moles/L x 5ml x 1L/10^3
ml x 40 g/mole = 0.4 g of NaOH
-
For a later step in isolating DNA, another cell biology student has to
make up a 5 mM solution of NaCl. He weighs out the very last grains
of NaCl and has 10 ug. NaCl MW = 58 g/mole. What volume of
water, in ml, does he need to make his NaCl solution?
5 mMoles/L x 1mole/10^3 mMoles = 0.005
moles/L
1/0.005 moles/L x 1/58 g/mole
x 0.00001 g = 3.45 x 10^-5 L = 3.45 x 10^-2 ml
-
Design a serial dilution given that you start with 5 M NaCl
and a final concentration of 250 uM.
-
To start with, figure out how many orders of magnitude
(multiples of 10) you will need to dilute by. Since you're going
from M to uM, you will need an order of 10^-6.
-
Next, what will be good multiples to do this in.
You don't want too many tubes, but you also don't want a volume so small
you can't pipet it. So let's try a serial dilution in 3 tubes.
-
Now, if you have three tubes with a TOTAL dilution
of 10^-6, then each tube should be a dilution of 10^-2.
-
Okay, now draw your diagram for clarification.
We're half way there.
 The way I have it now, our final concentration is
5 uM. We want 250 uM, so this way is too much.
The way I have it now, our final concentration is
5 uM. We want 250 uM, so this way is too much.
-
So we diluted too much. Let's decrease our first
two dilutions to 1/10 and see what happens.

-
We are very close. All we need now is a 1:2
dilution. This is the final serial dilution.
 There are other ways to do this. As long as
you come up with 250 uM in the end and the volumes are something you can
pipet in lab, your scheme is a success.
There are other ways to do this. As long as
you come up with 250 uM in the end and the volumes are something you can
pipet in lab, your scheme is a success.
-
What is the initial concentration in Mole/L?
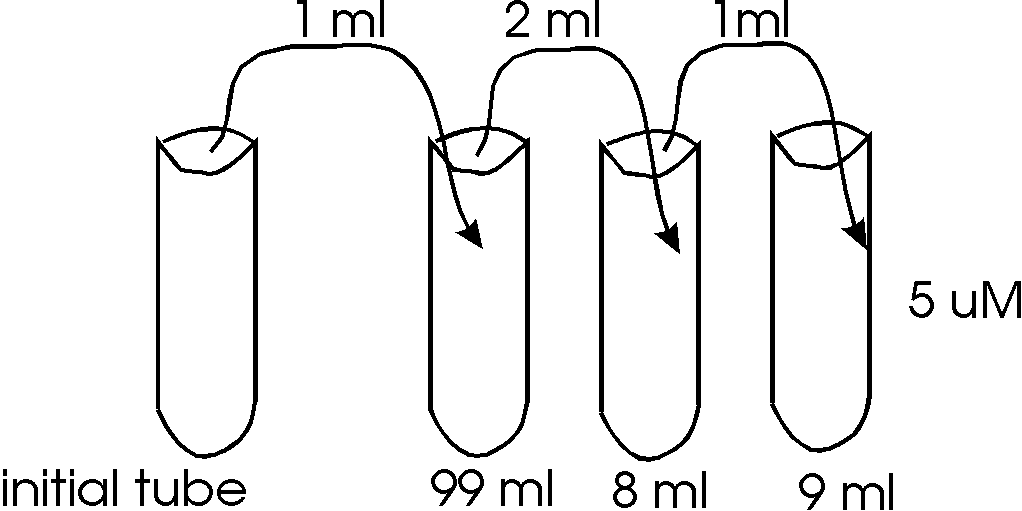 First, determine the dilution in each
tube:
First, determine the dilution in each
tube:
1/100
2/10 = 1/5
1/10
Now determine the TOTAL dilution:
1/100 x 1/5 x 1/10 = 1/5000
The dilution factor (DF) = 5000
so if Ci = DF x Cf
Ci = 5000 x 5 uM
Ci = 25000 uM = 0.025 M
-
A biology grad student has a 1 M solution of a chemical called DTT.
She needs to do a dilution and comes to a cell biology student for help.
She wants you tell her what volume of 1 M DTT to use in order to have 100
ul of 100 mM DTT. What do you tell her? Feeling noble, you
also tell her how much diluent (water) to use (the volume in ul).
First, determine what order of magnitude the dilution
will be. Here we're going from 1 M to 100 mM = 1 x 10^-3 M.
If the difference is less than (or equal to) 2 orders of magnitude, then
use the equation
C1V1 = C2V2
C1 = 1 M
C2 = 100 mM = 0.1 M
V1 = ?
V2 = 100 ul
Now, V1 = C2V2/C1
V1= (0.1 x 100)/1
V1 = 10 ul of 1 M DTT
Which means, 100 - 10 = 90 ul of water
-
What is the dilution if you put 250 ul of
a solution into a total volume of 5 ml?
First, get all the volumes in the same units.
So, 5 ml = 5000 ul or 250 ul = 0.25 ml.
Now: 250 ul/5000ul
= 1/20
That's the dilution. It's a straightforward
answer, and you DO NOT need to know concentrations.



